Bone Vasculature using a Stroke Model
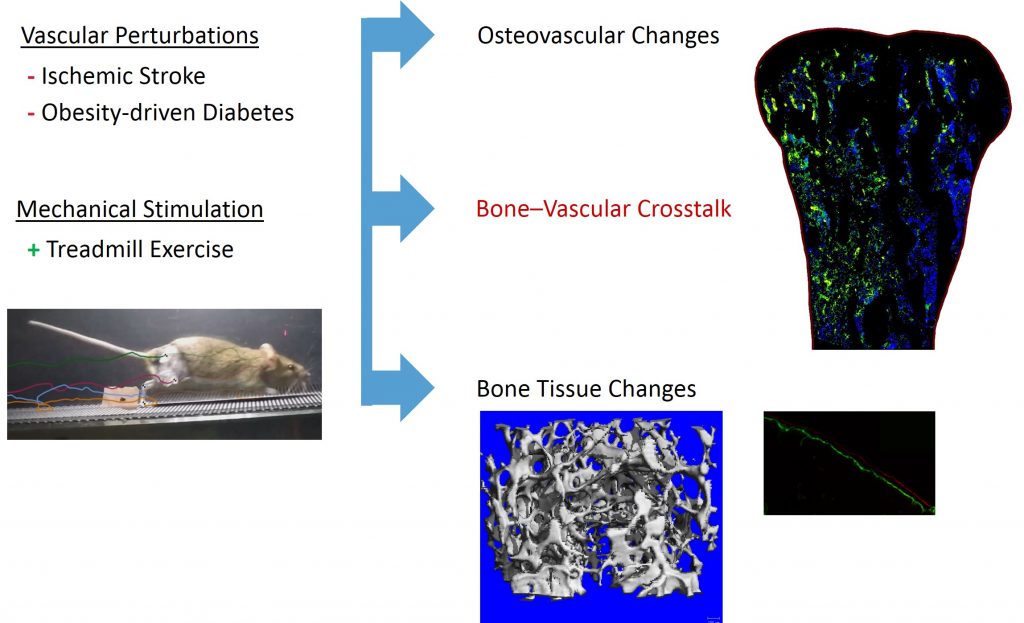
Adequate blood perfusion within bone is required to regulate and maintain the complex, metabolically expensive process of healthy bone remodeling. Several vascular degenerative diseases are correlated with increased risk of bone fracture, but the structural and biochemical changes to vasculature within bone are unknown. Our lab utilizes animal models of these lifestyle diseases to modulate the osteovascular environment, elucidating the pathophysiologic link between bone and vascular health. We combine these disease models with exercise and other pro-vascular rehabilitative strategies to determine the efficacy of potential treatments.
Changes in Bone-Vascular Interactions during Stroke Rehabilitation
Stroke is the second-most common cause of disability worldwide and the most common cause of disability in North Carolina, the ‘belt buckle’ of America’s stroke belt. Ischemic stroke victims experience up to 17% loss in bone mineral density, leading to 2-4 fold increase in fracture risk within the first 12 months. Bone is rapidly resorbed and bone quality is greatly reduced during the acute recovery period, which varies between patients and stroke severity but usually lasts for a couple of weeks. Exercise rehabilitation is usually initiated after the acute phase of stroke recovery. Although bed rest and limb disuse may account for some bone loss, stroke may cause blood supply changes to the bone as well. Adequate blood perfusion within bone is required to regulate and maintain the complex, metabolically expensive process of healthy bone remodeling. Exercise during the acute recovery period is known to increase blood perfusion in the brain via the eNOS/VEGF angiogenesis pathway, but the impact of blood perfusion in bone after stroke remains unexplored.
We use a mouse stroke model to investigate changes in bone health and blood perfusion during acute recovery. We determine the effectiveness of mechanics (exercise, neuromuscular stimulation) in maintaining osteovascular health by examining structural, biochemical, and functional changes in bone and vasculature. Our findings will elucidate the effects of stroke on the vascular system within bone and the potential benefit of physical and pharmaceutical rehabilitation interventions to the osteovascular system in human stroke survivors.
Progression of Metabolic Disease and Diet-Induced Obesity on the Osteovascular Environment
In addition to cardiovascular problems, for example peripheral artery disease and atherosclerosis, individuals with obesity and metabolic disease experience increased limb fracture rates despite greater bone mass than healthy individuals. Higher fracture rates may indicate vascular or bone quality deficiencies. We hypothesize that vascular changes with obesity are systemic and play a key role in fracture incidence in this population.
We feed mice a high fat diet, 60%, to initiate metabolic disease and obesity. During the progression of these two pathologies we examine bone and vascular properties with and without exercise interventions. The findings of this study will increase the understanding of the determinants of decreased bone fracture resistance due to dietary lifestyle disease.
Selected Publications and Presentations:
- Stangeland-Molo S, Britt CE, Smith TL, Danelson KA, Cole JH (2020) Examining contributions of limb unloading to changes in bone perfusion with ischemic stroke. Biomedical Engineering Society Annual Meeting, San Diego, CA, Oct 14-17, Submitted.
- Stangeland-Molo S, Hanne NJ, Khodjaniyazova S, Muddiman MC, Cole JH (2020) Examining changes in bone perfusion and bone-specific biomarkers with ischemic stroke. Orthopaedic Research Society Annual Meeting, Phoenix, AZ, Feb 8-11, Poster #1675.
- Hanne NJ, Steward AJ, Cox JM, Easter ED, Thornburg HL, Sessions MR, Pinnamaraju SV, Cole JH. High fat diet-induced obesity negatively affects whole bone bending strength but not cortical structure in the femur. bioRxiv preprint. [DOI: 10.1101/729624].
- Hanne NJ, Steward AJ, Geeroms C, Easter ED, Thornburg HL, Kerckhofs G, Parac-Vogt Tatjana, Sheng H, Cole JH. Ischemic stroke reduces bone perfusion and alters osteovascular structure. bioRxiv preprint. [DOI: 10.1101/729632].
- Hanne NJ, Easter ED, Stangeland-Molo S, Cole JH (2020). A minimally invasive technique for serial intraosseous perfusion measurements in the murine tibia using laser Doppler flowmetry. MethodsX 7:100814. DOI: 10.1016/j.mex.2020.100814. PMCID: PMC7082608.
- Hanne NJ*, Easter ED*, Cole JH (2019). Minimally invasive laser Doppler flowmetry is suitable for serial bone perfusion measurements in mice. Bone Reports 11:100231. DOI: 10.1016/j.bonr.2019.100231. PMCID: PMC6900537. * co-first authors
- Khodjaniyazova S, Hanne NJ, Cole JH, Muddiman DC (2019). Mass spectrometry imaging (MSI) of fresh bones using infrared matrix-assisted desorption electrospray ionization (IR-MALDESI). Analytical Methods 11(46):5929-5938. DOI: 10.1039/c9ay01886g.
- Hanne NJ, Steward AJ, Sessions MR, Thornburg HL, Sheng H, Cole JH (2019). Stroke prevents exercise-induced gains in bone microstructure but not composition in mice. ASME. Journal of Biomechanical Engineering 141(12):121008, Invited Award Paper. DOI: 10.1115/1.4045113. PMID: 31596925.
- Steward AJ, Cole JH, Ligler FS, Loboa EG (2016). Mechanical and vascular cues synergistically enhance osteogenesis in human mesenchymal stem cells. Tissue Engineering: Part A 22(15-16):997-1005. DOI: 10.1089/ten.TEA.2015.0533. PMCID: PMC4991610.
These projects were supported by the National Institutes of Health (K12 HD073945), the American Heart Association (17GRNT33710007), the American Society of Biomechanics (Junior Faculty Research Award), and the NC State Office of Undergraduate Research.