Bone Vasculature
Examining Bone Vasculature using a Bone on Chip Model
Collaboration with Dr. Michael Daniele, UNC/NC State BME Department, NC State Electrical and Computer Engineering Department
[You can find our previous research using a stroke model to study bone vasculature, here]
Three dimensional (3D) in vitro platforms have key advantages over in vivo models for discovery-based research. Besides lower cost, 3D in vitro platforms (microdevices and on-a-chip systems) allow decoupling of local and systemic effects on cell behavior and fine control of experimental conditions to reduce confounding effects. Microdevice platforms also recapitulate cell microenvironments, including maintenance of multiple cell types, which provides more physiologically relevant data and can be used for high-throughput screening. Microdevices have been used to recreate and gain deeper understanding of a variety of tissues. Our lab combines organic scaffold synthesis and principles of cell biology to develop a novel 3D bone-on-chip device. This device will be used to investigate bone and vascular cell interactions during inflammation seen after stroke. Bone is a hierarchical structure composed of cells, protein, and mineral. Cancellous bone has a porous architecture that aids in its load-directing function. Additionally, cancellous bone is in close proximity to blood vessels and is the largely affected by bone loss driven by pathology. Cancellous bone scaffolds for in vitro applications could greatly improve our understanding of bone loss during disease in controlled experiments. Cells respond more favorably when grown on a substrate that is similar to their native extracellular matrix. We hypothesize that osteoblasts cultured on biomimetic scaffolds will behave and respond to stimuli more like they would in the body. We focus on the effect of processing conditions on scaffold architecture, strength, and biocompatibility. We combine collagen and chitosan protein solutions and then use a modified freeze-drying process to create porosity within these protein blends. To incorporate mineral into the structure we passively mineralize in solution. We then determine scaffold composition, porosity, modulus, and biocompatibility. Results from this will determine the optimal processing conditions that will be use in the bone-on-chip device Mineralized bone scaffold Representative scanning electron micrograph of scaffold porosity 3D reconstruction of scaffold (tan) with segmented porosity visible (teal = pore, yellow = scaffold) Microdevices provide an excellent opportunity to study complex cell interactions in a highly controlled manner. Motivated by our work in a mouse stroke model, we are interested in better understanding bone loss after ischemic stroke using a microdevice. Inflammatory cytokines (IL-6, IL-1β, and IFN-γ) have been detected in stroke patient serum and are elevated from hours to months after the acute event. Inflammation is important in wound healing and when controlled promotes bone growth. However, chronic inflammation, as seen in osteoarthritis, can damage bone and reduce bone mass. Our hypothesis is that the prolonged elevated levels of inflammatory factors post-stroke elicit a chronic inflammatory response leading to bone mass loss. We are developing a bone-on-chip device that incorporates biomimetic bone scaffolds and relevant cell types to provide a physiologic model of cytokine delivery and cell response within bone. We leverage basic biology to determine cell-cell signaling and gene program changes, as well as computational fluid dynamic models to inform geometry and flow rates necessary for proper nutrient transport and fluid shear. Findings from our work will determine cell behavior changes in this environment with the ultimate goal of identifying targets for more effective clinical treatment. Schematic and images of bone-on-chip device
Biomimetic bone scaffold
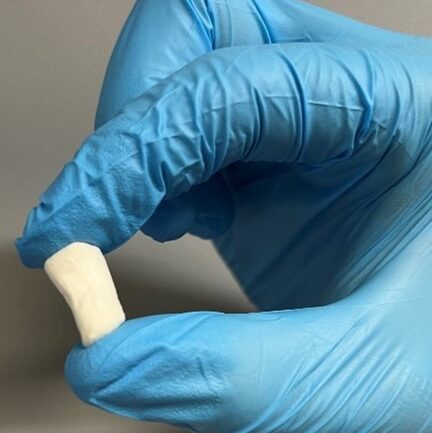
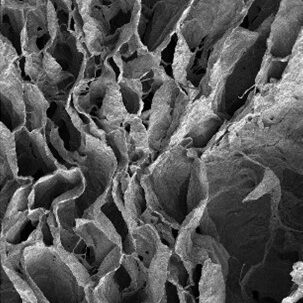

Bone-on-chip device to study bone-vascular interactions
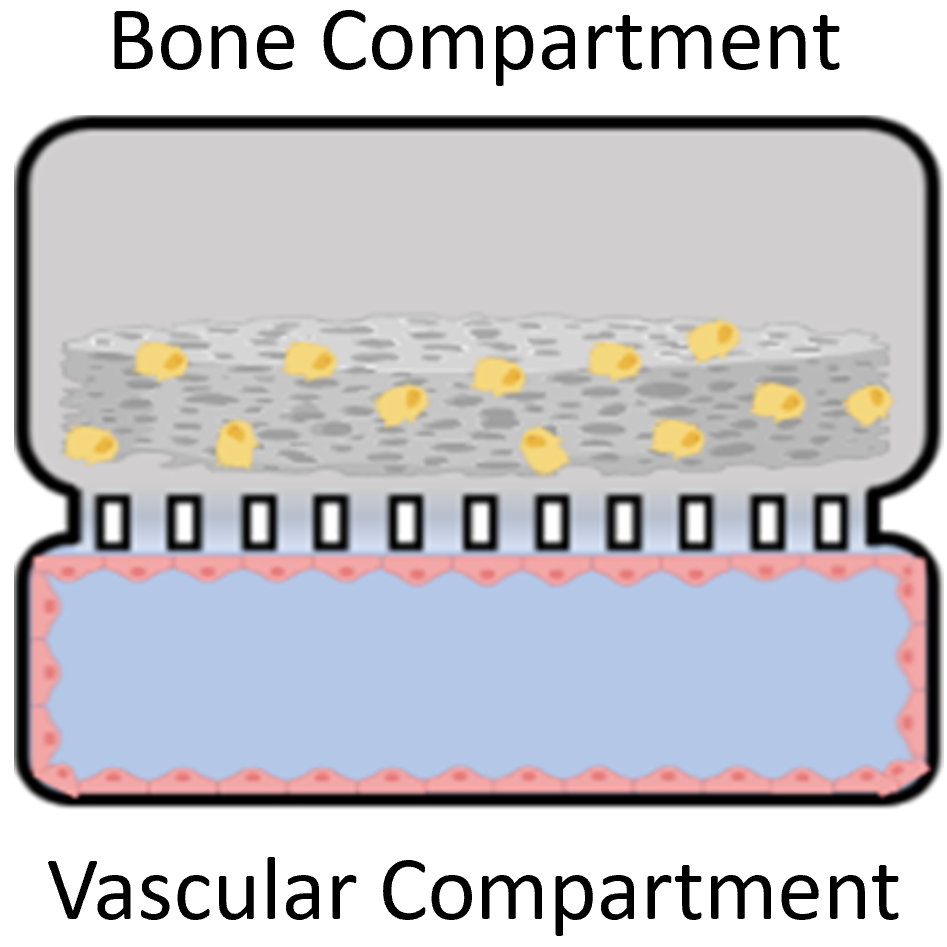
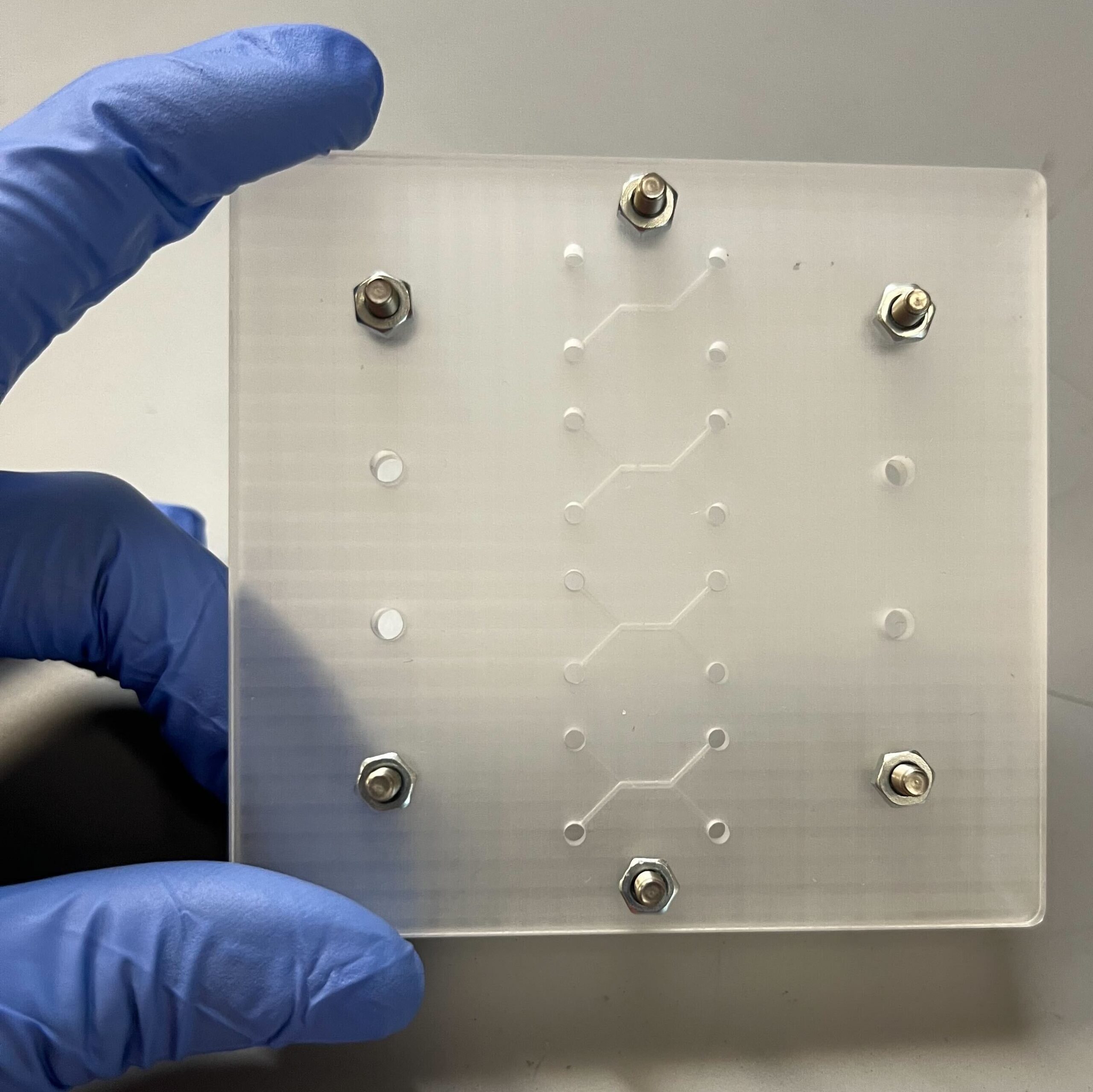
Presentations and Publications:
